Unique Protocol ID | SOC-3 |
ClinicalTrials.gov ID | ClinicalTrials.gov Identifier: NCT03983226 |
Version Number | 2.0 |
IRB ID | B2018-125R
Institutional Review Board of Zhongshan Hospital, Fudan University
ec@zs-hospital.sh.cn, 021-64041990转3257 |
Data Monitoring Committee | Yes |
Brief Title | Surgery and Niraparib in Secondary Recurrent Ovarian Cancer (SOC-3) |
Official Title | A phase II, randomized study of cytoreductive surgery combined with niraparib maintenance in platinum-sensitive, secondary recurrent ovarian cancer |
Study Type | Interventional |
Secondary IDs | SGOG OV5 |
Responsible Party | Shanghai Gynecologic Oncology Group |
Sponsor | Shanghai Gynecologic Oncology Group |
Collaborators | Fudan University
Shanghai Jiao Tong University School of Medicine
Zhejiang Cancer Hospital
Sun Yat-sen University |
Brief Summary | The purpose of this study is to evaluate the role of cytoreductive surgery and Niraparib maintenance in platinum-sensitive secondary recurrent ovarian cancer. |
Detailed Description | This exploratory trial is to compare the efficacy of secondary cytoreductive surgery followed by chemotherapy and Niraparib maintenance, versus chemotherapy alone followed by Niraparib maintenance in patients with platinum-sensitive secondary recurrent ovarian cancer who never received secondary cytoreduction when recurrent. |
Study Start Date | OCT 2019 |
Primary Completion Date | JUNE 2023 (final data collection date for primary outcome measure) |
Study Completion Date | DEC 2024 (Final data collection date for study.) |
Study Design | Allocation: Randomized
Endpoint Classification: Safety/Efficacy Study
Intervention Model: Parallel Assignment
Masking: Open Label
Primary Purpose: Treatment |
Outcome Measures | Primary Outcome:
12-month non-progression rate
(Description: percentage of the patients without disease progression at 12-month follow-up)
(Time Frame: 12 months)
Secondary Outcome:
1.Progression-free survival
(from date of randomization until the date of 3rd relapse/progression or death (whatever occurs first)
Revise (patients without progression at 6-month after randomization)
(Up to 24 months after last patient randomized)
2.Treatment free intervals
(It is the total intervals of the ending date from system anticancer therapy to the starting date of the subsequent anticancer therapy or death, such as TFI1+TIF2+TFI3…)
(Up to 24 months after last patient randomized)
3.Overall Survival
(from date of randomization until the date of death from any cause or last follow-up)
(Approximately up to 24 months after last patient randomized)
4.30-day post-operative complications
(MSKCC surgical complications grading method and CTCAE v4.03 criteria will be adopted for evaluating the perioperative complications)
(From the operation until after 30 days)
5.Quality of life assessments (QLQ-C30, OV28, FACT-O)
The EORTC core quality of life questionnaire (QLQ-C30, version 3.0)
The EORTC core quality of life questionnaire (QLQ-OV28)
Functional Assessment of Cancer Therapy- Ovary (FACT-O)
(baseline; 6 and 12 months after randomization) |
Conditions | Ovarian Cancer Recurrent, Fallopian Tube Cancer, Primary Peritoneal Carcinoma |
Keywords: Recurrent Ovarian Cancer; Secondary Cytoreductive Surgery; Chemotherapy; Niraparib maintenance |
Arms | 1.Experimental: Surgery
Interventions:
Intervention/treatment: Tumor debulking surgery (surgery in recurrent ovarian disease)
Procedure: Maximum effort cytoreductive surgery combined with Niraparib maintenance;
Drug: platinum Salvage chemotherapy and Niraparib
2.Active Comparator: No surgery
Intervention: carboplatin/taxane, carboplatin/gemcitabine, cisplatin/gemcitabine, liposome doxorubicin/carboplatin-----
Drug: Salvage chemotherapy and Niraparib |
Intervention Description: | secondary cytoreductive surgery followed by 6 cycles of post-operative chemotherapy and Niraparib maintenance; versus 6 cycles of post-operative chemotherapy and Niraparib maintenance |
Interventions | 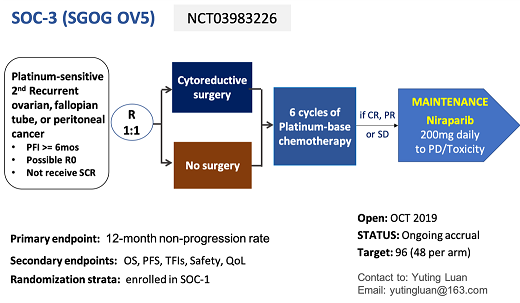
|
Eligibility Criteria | Inclusion Criteria:
•Age ≥18 years to ≤ 75 years
•ECOG performance status of 0 to 2
•Patients with platinum-sensitive, secondary relapsed epithelial ovarian, primary peritoneal, or fallopian tube cancer (EOC, PPC, FTC), which is defined as those with platinum-free interval of 6 months or more.
•Front-line or second-line treatment may have included maintenance therapy (i.e. bevacizumab, PARP inhibitor)
•Never received secondary cytoreductive surgery when recurrence
•Assessed by the experienced surgeons, complete resection of all recurrent disease is possible. Single or localized lesions identified by CT, or MRI, or PET/CT. PI and Co-PI reach consensus if extensive lesions or carcinomatosis.
•It can be included if single lesion outside the peritoneal cavity can be resected.
•No more than 3 disease lesions by central-reviewed PET/CT imaging if the participated center has never participated in any surgical trials on ovarian cancer before.
•Patients who have given their signed and written informed consent and their consent.
Exclusion Criteria:
•Patients with borderline tumors as well as non-epithelial tumors.
•Patients for interval-debulking, or for second-look surgery, or palliative surgery planned.
•Patients whom have already undergone secondary cytoreduction for recurrent disease are excluded.
•Impossible to assess the resectability. Radiological signs suggesting complete resection is impossible.
•Patients who have received more than two previous regimen of chemotherapy (maintenance is not considered a third regimen).
•Third relapse or more.
•Patients with second or other malignancies who have been treated by surgery, if the treatment might interfere with the treatment of relapsed ovarian cancer or if major impact on prognosis is expected.
•Progression during chemotherapy or recurrence within 6 months after second-line platinum-based therapy
•Any contradiction not allowing surgery or chemotherapy or Niraparib
•Accompanied by hypoxia serious chronic obstructive pulmonary disease
•Uncontrolled hypertension, cerebrovascular accident/ Stroke, myocardial infarct, unstable angina, untreated thrombosis, chronic congestive heart failure, or serious arrhythmia in need of medicine.
•Severe hepatitis, history of liver disease, nephrotic syndrome, renal insufficiency
•Active ulcer history, abdominal wall fistula, perforation of gastrointestinal tract, or Intra-abdominal abscess, or simultaneously apply treatment/prevent ulcers therapy.
•Uncontrolled diabetes
•Uncontrolled epilepsy need long-term antiepileptic treatment.
•Any medication induced considerable risk of surgery, e.g. estimated bleeding due to oral anticoagulating agents
•≥3 grade anemia, neutropenia or thrombocytopenia due to chemotherapy, and lasted for more than 4 weeks
•Patients with a known hypersensitivity to Niraparib or any of the excipients of the product |
Gender | Female |
Minimum Age | 18 Years |
Maximum Age | 75 Years |
Accepts Healthy Volunteers | No |
Sample size | 96 |
Central Contact | Rong Jiang, MD. +862164041990. jiang.rong@zs-hospital.sh.cn
Yuting Luan, RN. +862164041990. yutingluan@163.com |
Principal Investigators | Tingyan Shi, MD, PhD.
Tel: +862164041990. Email: shi.tingyan@zs-hospital.sh.cn |
Locations | China |
Recruitment Status | Recruiting |
 English
English
 English
English
 English
English