Unique Protocol ID | SGOG OV6 |
ClinicalTrials.gov ID | ClinicalTrials.gov Identifier: NCT04710797 |
Version Number | 2.0 |
IRB ID | 5010-2020-02-01 |
Data Monitoring Committee: | NA |
Brief Title: | Lymphadenectomy in early ovarian cancer |
Official Title: | A prospective randomized multicenter trial for lymphadenectomy in early-stage ovarian cancer |
Study Type: | multicenter, randomized controlled, phase III trial |
Secondary IDs |
|
Responsible Party: | Jihong Liu, Professor, Sun Yat-sen University |
Sponsor: | Sun Yat-sen University Clinical Research 5010 Program (No. 2020003). |
Collaborators: |
|
Brief Summary: | To assess the impact of comprehensive staging surgery with no lymphadenectomy onsurvival and quality of life in patients with early-stage ovarian cancer. |
Detailed Description: | OBJECTIVES: Compare the efficacy and safety in patients with InternationalFederation of Gynecology and Obstetrics (FIGO) stage IA -IIB epithelial ovarian cancer,undergo completion staging surgery including systematic pelvic and para-aorticlymphadenectomy versus comprehensive staging surgery without lymphadenectomy. OUTLINE: This is a randomized phase III multicenter study. Patients will receivecomprehensive staging surgery without Lymphadenectomy or completion stagingsurgery including systematic pelvic and para-aortic lymphadenectomy, and theadjuvant chemotherapy will accord to National Comprehensive Cancer Network(NCCN) guidelines. Patients are followed up every 3 months within the first 2 years,and then every 6 months. PROJECTED ACCRUAL: A total of 656 patients will be recruited for this study within 5 years. |
Study Start Date: | January 31, 2021 |
Primary Completion Date: | December 31, 2025 |
Study Completion Date: | December 31, 2028 |
Study Design: | Study Type:Interventional Estimated Enrollment:656 participants Allocation: Randomized Intervention Model: Parallel Assignment comprehensive staging surgery with or withoutlymphadenectomy Masking:None (Open Label) |
Outcome Measures: | Primary Outcome Measure: 1.PFS(Progression-free survival) [Time Frame: From date of randomization until thedate of first documented progression or date of death from any cause, whichevercame first] Secondary Outcome Measures: 1.OS(Overall Survival) [Time Frame: From date of randomization until the date of death from any cause or date of last follow up] 2.Recurrence rate of lymph node [Time Frame: 3 years] The recurrence rate in the retroperitoneal lymph nodes after primary surgery 3. QoL(Quality of life) [Time Frame: Baseline, 6 months and 1 year after surgery] |
Conditions: | Recruiting |
Keywords: | Lymph Node Dissection; Ovarian Cancer; Survival |
Arms: | Experimental group, Comparative group |
Intervention Description: | Patients assigned to experimental group will undergo comprehensive staging surgery, but lymphadenectomy. Patients assigned to comparative group will undergo completion staging surgery including systematic pelvic and para-aortic lymphadenectomy. |
Interventions: | 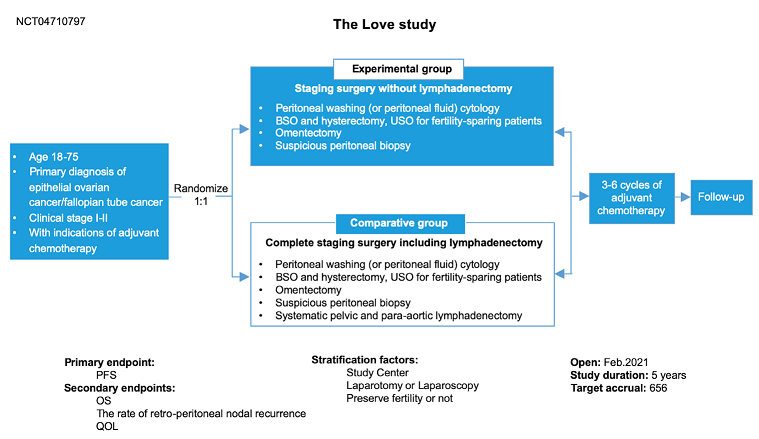 |
Eligibility Criteria: | Major inclusion criteria are pathologic confirmed stage IA–IIB EOC or fallopian tube carcinomas and patients have indications for adjuvant chemotherapy either confirmed by intraoperative fast frozen section or previous pathology after an incomplete staging surgery: High-grade serous carcinoma, Grade 3 endometrioid carcinoma, Clear cell carcinoma, Grade 2 endometrioid carcinoma in International Federation of Gynaecology and Obstetrics (FIGO) stage IC–IIB, Grade 1 endometrioid carcinoma and mucinous carcinoma in FIGO stage IIA–IIB; age 18 to 75 years; written informed consent provided; Eastern Cooperative Oncology Group performance status 0 to 1. If preoperative or intraoperative lymph node metastasis is suspected, intraoperative lymph node sampling and fast frozen pathology must be performed, only negative results can be randomized. Major exclusion criteria are non-epithelial ovarian tumors and low-grade carcinoma; severe rectum involvement which lead to partial rectum resection; secondary invasive neoplasms in the last 5 years other than synchronal endometrial carcinoma FIGO IA G1/2 or non-melanoma skin cancer or breast cancer T1N0M0 G1/2 (without any signs of relapse or activity); prior chemotherapy for ovarian cancer or abdominal/pelvic radiotherapy; diseases of the lymph system (including lymph edema of unknown origin); prior retroperitoneal lymph node dissection (systematic or sampling). |
Gender: | Female |
Minimum Age: | 18 |
Maximum Age: | 75 |
Accepts Healthy Volunteers: | No |
Sample size | 656 |
Central Contact: | Jihong Liu, Ph.D,professor 86-20-87343102 liujih@mail.sysu.edu.cn Ting Deng, Ph.D. 86-20-87343105 dengting@sysucc.org.cn |
Principal Investigators | Jihong Liu, Ph.D. Sun Yat-sen University |
Locations: | Department of Gynecologic Oncology, Sun Yat-sen UniversityCancer Center Recruiting Guangzhou, Guangdong, China, 510060 |
Recruitment Status: | Recruiting |
 English
English
 English
English
 English
English